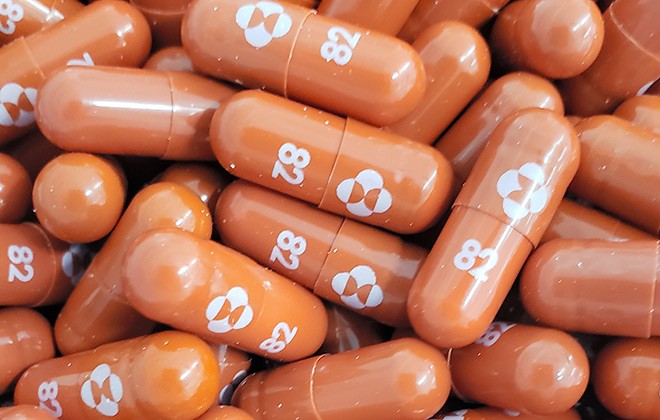
The European Medicines Agency will begin reviewing data on game-changing antiviral pill molnupiravir, produced by US company MSD, against COVID-19, to support countries who want to use this medicine before receiving final authorisation.
In a statement, EMA and the Heads of Medicines Agencies (HMA) of the EU’s member states announced they have agreed on the need for additional guidance on COVID-19 treatments in light of rising infection rates and deaths across Europe.
EMA is reviewing available data on the use of molnupiravir (also known as MK 4482 or Lagevrio) to support national authorities who may decide on using this medicine for COVID-19 treatment before its authorisation.
While the more comprehensive rolling review is ongoing ahead of a possible application for marketing authorisation, EMA’s Committee for Medicinal Products for Human Use (CHMP) will provide EU-wide recommendations in the shortest possible time frame to help national authorities decide possible early use of the medicine.
Molnupiravir is an oral antiviral medicine developed by US company Merck (known as MSD in the EU) in collaboration with Ridgeback Biotherapeutics.
It reduces the ability of SARS CoV 2 (the virus that causes COVID-19) to multiply in the body.
It does this by increasing the number of alterations (mutations) in the virus’s genetic material (RNA) in a way that impairs the ability of SARS-CoV-2 to multiply.
EMA and the HMA said they remain committed to expediting the evaluation of COVID-19 treatments and vaccines while ensuring these meet the EU’s safety and efficacy standards.
Last week, Britain became the first country in the world to approve the COVID-19 antiviral pill in a boost to the fight against the pandemic.
Britain’s Medicines and Healthcare products Regulatory Agency (MHRA) recommended the drug, molnupiravir, for use in people with mild to moderate COVID-19 and at least one risk factor for developing severe illness, such as obesity, older age diabetes, and heart disease.
The regulator said it would be administered following a positive COVID-19 test and within five days of the onset of symptoms.
The green light is the first for an oral antiviral treatment for COVID-19 and a COVID-19 drug that will be administered widely in the community.
US advisers will meet on November 30 to review the drug’s safety and efficacy data and vote on whether molnupiravir should be authorised.
The pill, which will be branded as Lagevrio in Britain, is designed to introduce errors into the genetic code of the coronavirus that causes COVID-19 and is taken twice a day for five days.
MSD said animal testing shows that molnupiravir is safe, but the data have not yet been made public.
Treatments to tackle the pandemic, which has killed more than 5.2 million people worldwide, have so far focused mainly on vaccines.
Other options, including Gilead’s infused antiviral remdesivir and generic steroid dexamethasone, are generally only given after a patient has been hospitalised.
The speedy approval in Britain, the first Western country to approve a COVID-19 vaccine, comes as it struggles to tame soaring infections.