The European Medicines Agency (EMA) has approved another COVID-19 booster vaccine adapted by BioNTech-Pfizer (Comirnaty) that targets BA.4 and BA.5 subvariants of the Omicron variant. “It is another key step in supporting Member
The European Medicines Agency (EMA) has recommended using two COVID-19 vaccines adapted against the Omicron variant by Pfizer/BioNTech and Moderna. The so-called Comirnaty Original/Omicron BA.1 (from Pfizer/BioNTech) and Spikevax Bivalent Original/Omicron BA.1
The European Medicines Agency (EMA) has started a rolling review for a Spikevax (Moderna) vaccine adapted to provide better protection against specific variants of SARS-CoV-2. The Ministry of Health said the review
The European Medicines Agency (EMA) has started a rolling review for a version of Comirnaty adapted to provide better protection against a specific variant or variants of SARS-CoV-2, the virus that causes COVID-19.
The European Medicines Agency (EMA) announced its human medicines committee (CHMP) started a rolling review of VLA2001, a COVID-19 vaccine developed by French company Valneva. The CHMP’s decision to start the rolling
The European Medicines Agency (EMA) has recommended the use of Comirnaty, the COVID-19 vaccine produced by BioNTech/Pfizer, be extended to children aged between 5 and 11. Cyprus has already decided it will
The European Medicines Agency (EMA) has started evaluating an application for conditional marketing authorisation for Novavax’s COVID-19 vaccine, Nuvaxovid, the agency announced. The EU assessment will proceed under an accelerated timeline. An
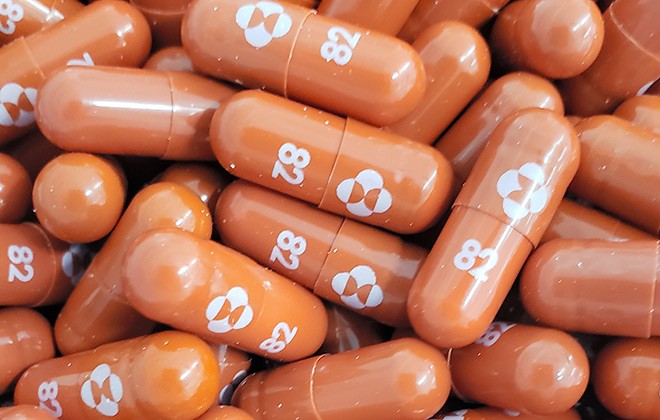
The European Medicines Agency will begin reviewing data on game-changing antiviral pill molnupiravir, produced by US company MSD, against COVID-19, to support countries who want to use this medicine before receiving final
With the Delta variant-powered COVID-19 infection wave crippling the EU, European medicine authorities urge unvaccinated people to get a jab before contracting the virus. The European Medicines Agency (EMA), in a joint
Cyprus will use the Pfizer/BioNTech COVID-19 vaccine on 12 to 15-year-olds when and if approved by the European Medicines Agency (EMA), said deputy director of Pharmaceutical Services Elena Panayiotopoulou. The Health Ministry